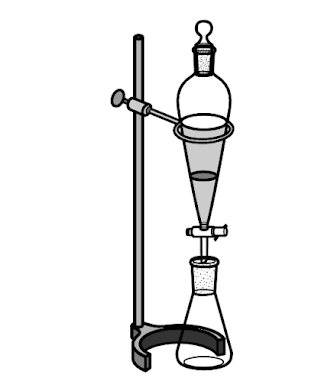
Extraction involves dissolving a compound or compounds either (1) from a solid into a solvent or (2) from a solution into another solvent. Acid-base extraction is the process of separating acids and bases in an organic mixture.
Acid-base extraction is typically used to separate organic compounds from each other based on their acid-base properties.
Generally, most organic compounds are neutral, and therefore more soluble in organic solvents than they are in water. However, if the organic compound becomes ionic, then it becomes more soluble in water. This is useful in extracting an organic acid or base compound from an organic phase to an aqueous phase.
An acid-base extraction can be used to extract carboxylic acids from the organic layer into the aqueous layer. Most organic carboxylic acids are insoluble or slightly soluble in water, but these compounds are highly soluble in dilute aqueous sodium hydroxide because the acid is deprotonated by the base producing the sodium carboxylate salt.
Acid-Base Extraction
The primary goal of food is to promote our health and general well-being. Food science entails comprehending the characteristics, composition, and behaviors of food constituents in different situations, such as storage, handling, and consumption.
October 9, 2022
The Most Popular Posts
-
Resveratrol is a naturally occurring polyphenol found in a range of plants, particularly in the skin of red grapes, blueberries, raspberries...
-
Navel oranges are not only delicious but also packed with numerous nutritional benefits that contribute to overall health and well-being. T...
-
Crude fat is the term used to refer to the crude mixture of fat-soluble material present in a sample. Crude fat also known as the ether ext...
-
Gelatinization occurs when starch granules are heated in a liquid. It is responsible for the thickening of food systems. The process is an i...
-
Crude fiber is a measure of the quantity of indigestible cellulose, pentosans, lignin, and other components of this type in present foods. ...